40 phase labels in chemical equations
Phase Rule - Teaching Phase Equilibria Gibbs Phase Rule is expressed by the simple formulation: P + F = C + 2, where P is the number of phases in the system A phase is any physically separable material in the system. Every unique mineral is a phase (including polymorphs); igneous melts, liquids (aqueous solutions), and vapor are also considered unique phases. › 32454429 › Harris_QuantitativeHarris Quantitative Chemical Analysis 8th edition - Academia.edu Harris Quantitative Chemical Analysis 8th edition (PDF) Harris Quantitative Chemical Analysis 8th edition | David Garcia - Academia.edu Academia.edu uses cookies to personalize content, tailor ads and improve the user experience.
Symbols in Chemical Equations - Harper College Symbol: Meaning + used to separate one reactant or product from another used to separate the reactants from the products - it is pronounced "yields" or "produces" when the equation is read
Phase labels in chemical equations
saylordotorg.github.io › text_the-basics-ofOxidation-Reduction (Redox) Reactions - GitHub Pages The half reaction for the oxidation reaction, omitting phase labels, is as follows: Zn → Zn 2+ + 2e −. This half reaction is balanced in terms of the number of zinc atoms, and it also shows the two electrons that are needed as products to account for the zinc atom losing two negative charges to become a 2+ ion. Factor-Label Method in Chemistry: Definition, Examples & Practice ... The Factor-Label Method. Believe it or not, one simple method can be used to accomplish many of the basic calculations in chemistry. The method does not involve years of calculus courses or other ... 3 Steps for Balancing Chemical Equations - ThoughtCo A chemical equation describes what happens in a chemical reaction. The equation identifies the reactants (starting materials) and products (resulting substances), the formulas of the participants, the phases of the participants (solid, liquid, gas), the direction of the chemical reaction, and the amount of each substance. Chemical equations are balanced for mass and charge, meaning the number and type of atoms on the left side of the arrow is the same as the number of type of atoms on the ...
Phase labels in chemical equations. 1.2 Phases and Classification of Matter - Chemistry The three most common states or phases of matter are solid, liquid, and gas. A fourth state of matter, plasma, occurs naturally in the interiors of stars. A plasma is a gaseous state of matter that contains appreciable numbers of electrically charged particles ( Figure 2 ). › createJoin LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols; kitchingroup.cheme.cmu.edu › pycse › pycsepycse - Python3 Computations in Science and Engineering Note that only one root is real (and even then, we have to interpret 0.j as not being imaginary. Also, in a cubic polynomial, there can only be two imaginary roots). In this case that means there is only one phase present. PDF Lecture Notes | Fundamentals of Materials Science | Materials Science ... Heat Storage and Release in Phase Transitions (PDF - 1.1 MB) (PDF - 2.1 MB) L6 The Hydrogen Atom (cont.) Examples of Work Important in Materials Science and Engineering: Polarization, Magnetic, Chemical L7 Alphabet Soup: The Periodic Table Thermal Properties of Materials; Fundamental Equations (PDF - 3.9 MB)
Conventions for Writing Chemical Equations - Arrows, Phases ... Conventions for Writing Chemical Equations - Arrows, Phases, Coefficients and more - YouTube. Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2NaHCO3 (s) − →−200°C Na2CO3 (s) +CO2(g) +H2O(ℓ) Key Takeaways Many chemical equations also include phase labels for Many chemical equations also include phase labels for. School Eastern Visayas State University - Tacloban City Main Campus; Course Title AGRICULTUR 847,272; Uploaded By MasterPencil4687. Pages 46 This preview shows page 25 - 27 out of 46 pages. ... Solved 1. Write balanced chemical equations using proper | Chegg.com 1. Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron (III) oxide, also known as rust.
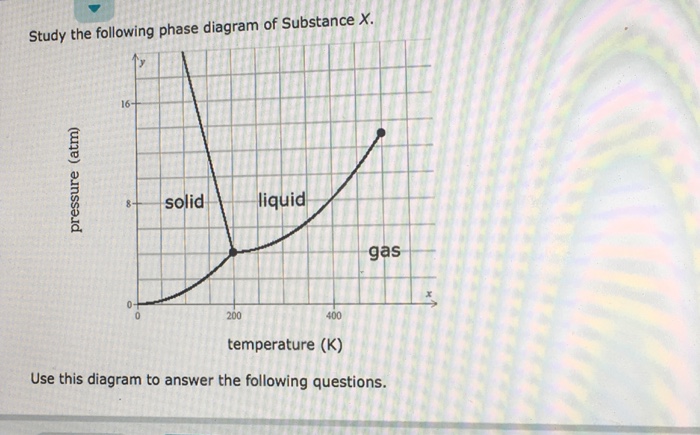
Chemical Equations - GitHub Pages It is not uncommon to include a phase label with each formula— (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H2(g) + O2(g) → H2O (ℓ) Note The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example: 2NaHCO 3 (s) → 200°C Na 2 CO 3 (s) + CO 2 (g) + H 2 O(ℓ) Phases in chemical reactions | StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l) Phase Diagrams - Chemistry - University of Hawaiʻi Using the phase diagram for water given in [link], determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 °C and 0.3 kPa (f) 50 °C and 0.3 kPa Solution
A thermochemical equation is a balanced chemical reaction equation ... A thermochemical equation is a balanced chemical reaction equation (including phase labels) with the enthalpy of reaction value written directly after the equation THERMOCHEMICAL EQUATIONS A reaction that produces heat is referred to as exothermic. For exothermic reactions, enthalpy values are assigned a negative value (-DH).
GCSE CHEMISTRY - What are State Symbols? - (s) - (l) - (g) - (aq ... The complete equations in words and symbols would be. potassium + chlorine potassium chloride. 2 K (s) + Cl 2(g) 2 K Cl (s) lith ium + oxygen lithium oxide. 4 Li (s) + O 2 (g) 2 Li 2 O (s) The state symbols in brackets show the physical state of. the substance at the reaction temperature.
Net Ionic Equations | Chemistry for Non-Majors | | Course Hero Write a balanced net ionic equation for this reaction. Step 1: Plan the problem . Write and balance the molecular equation first, making sure that all formulas are correct. Then write the ionic equation, showing all aqueous substances as ions. Carry through any coefficients. Finally, eliminate spectator ions and write the net ionic equation.
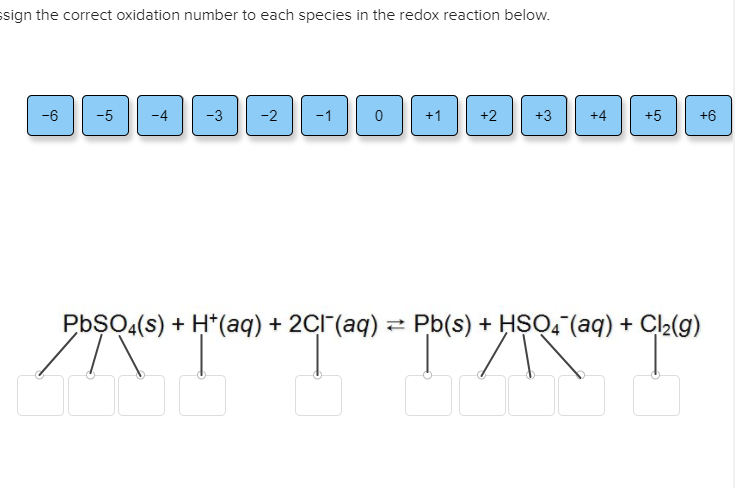
30 Label Each Element In The Chemical Reaction Below With The Correct Oxidation State. - Label ...
What are Chemical Equations? Detailed Explanation, Examples The reactants and the products (for which the chemical formulae are written in chemical equations) can be separated by one of the following four symbols. In order to describe a net forward reaction, the symbol '→' is used. In order to describe a state of chemical equilibrium, the symbol '⇌' is used.
Phase plane - Wikipedia Phase plane. In applied mathematics, in particular the context of nonlinear system analysis, a phase plane is a visual display of certain characteristics of certain kinds of differential equations; a coordinate plane with axes being the values of the two state variables, say ( x, y ), or ( q, p) etc. (any pair of variables).
en.wikipedia.org › wiki › Lagrangian_mechanicsLagrangian mechanics - Wikipedia Suppose there exists a bead sliding around on a wire, or a swinging simple pendulum, etc.If one tracks each of the massive objects (bead, pendulum bob, etc.) as a particle, calculation of the motion of the particle using Newtonian mechanics would require solving for the time-varying constraint force required to keep the particle in the constrained motion (reaction force exerted by the wire on ...
› 32945832 › Quantitative_ChemicalQuantitative Chemical Analysis 7E Daniel C. Harris Quantitative Chemical Analysis 7E Daniel C. Harris. Alfredo Morales. Download Download PDF. Full PDF Package Download Full PDF Package. This Paper. A short summary of ...
PDF Strategy to Determine the Phase of a Chemical Mixture give the phase of the mixture? Well, you have to determine it using the temperature (T), pressure (P), and composition (or mole fractions) of the mixture. Given: T P Z where Z means z z z, , 12, , , nc. Find: … Ph or phase. nc is the number of components . Pure-Component Mixture (nc = 1, a pure compound ) For P = 1 atm, find T m and T
Phase diagram - Wikipedia A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium . Contents 1 Overview 2 Types 2.1 2-dimensional diagrams
How to Identify States of Matter in a Chemical Formula Chemical equations and the formulas of substances that appear in them are powerful recipes for laboratory experiments, and information about the state of matter of each reactant and product is vital. If you are only told that H2O is a reactant, you will not know whether to add ice -- the solid form -- or liquid water or steam -- the gaseous form.
Chemical Reactions and Equations - GitHub Pages In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products. ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as ...
Using state symbols in chemical equations - BBC Bitesize Learn about and revise equations and chemical reactions with this BBC Bitesize GCSE Combined Science (OCR 21C) study guide.
Phase Definition and Examples - ThoughtCo In chemistry and physics, a phase is a physically distinctive form of matter, such as a solid, liquid, gas, or plasma. A phase of matter is characterized by having relatively uniform chemical and physical properties. Phases are different from states of matter.
SOLVED:16. Write a balanced equation (including phase labels) for the ... we are just looking to balance some chemical equations. So the first one we have a gcl in the solid state. I won't list. I went right down the states but I will say them aloud just to save a bit of times what a gcl solid at two and H. Three A quits. That gives us A G. And H. 32 plus acquis Art C. L minus liquids. The next example we've got C R E N two cl two cl acquits R two H 20.
Solved Write the balanced chemical equations, including | Chegg.com Science; Chemistry; Chemistry questions and answers; Write the balanced chemical equations, including phase labels, for the precipitation reaction that occurs when HCaq) is added to a solution containing mercury U) ions: 0 a) Hg22+(s) + 2HCl(aq) → 2H+(aq) + Hg2C12(s) 0 b) H822+ (aq) + 2H2 + 2C12(aq) → 2H+(aq) + Hg2Cl2(s) c) H822+ (aq) + 2HCO) → 2H+ (aq) + Hg202(s) O d) H822+ (aq) +2HCNaq ...

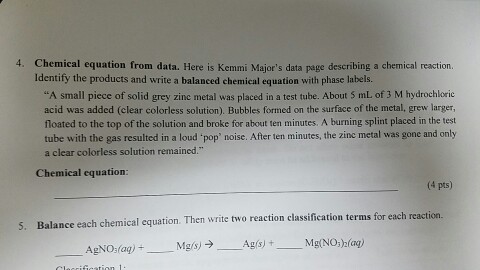
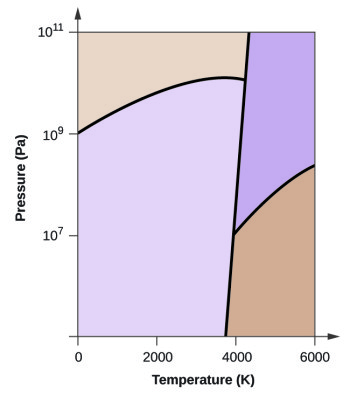

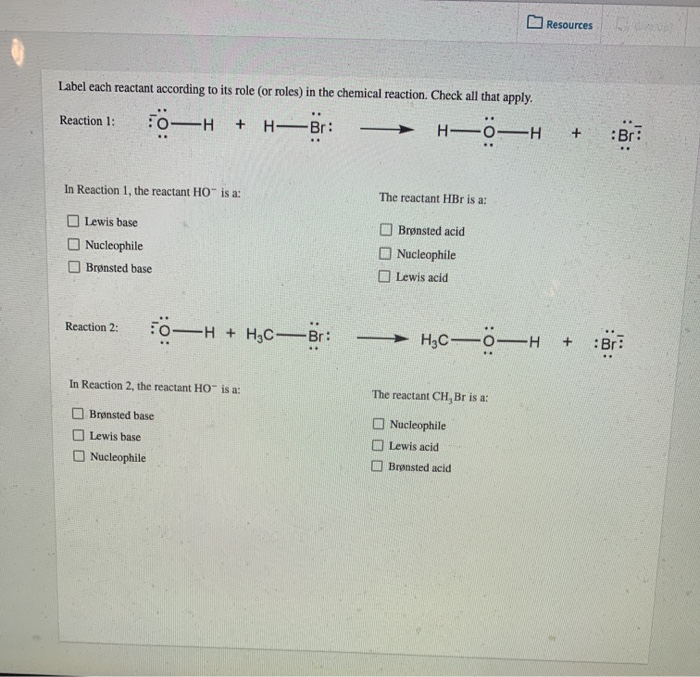
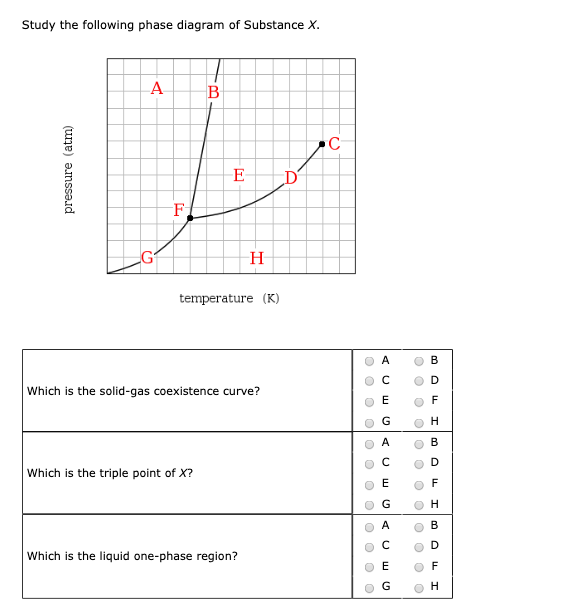
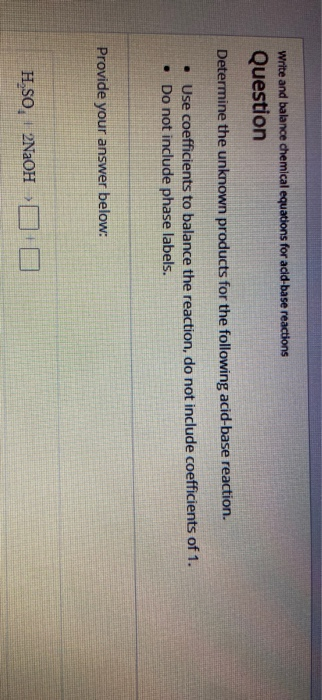
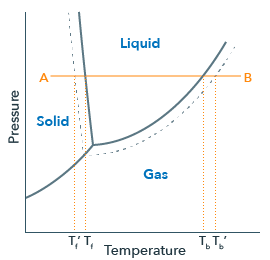

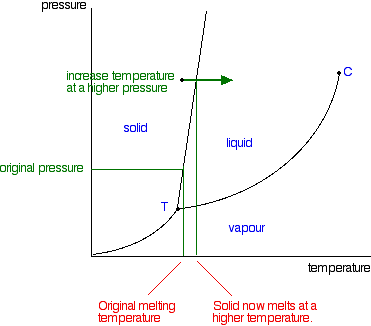
Post a Comment for "40 phase labels in chemical equations"